Technologies
1/3
Research, Development & New Technologies
Recent advances in cell reprogramming technologies open up new possibilities for generation of stem cell lines and specific differentiated cell types from patient own somatic cells that can be used for disease modeling, toxicity screening, drug discovery, metabolism studies and as cellular therapeutics for multiple diseases and disorders. Over the last decades several cell reprogramming methods such as nuclear transfer, cell fusion and transfection or transduction with pluripotent factors have been developed.
However, the applicability for disease modeling, drug/toxicity screening and cell therapy of reprogrammed cells by these approaches has been limited by the inefficiency of reprogramming methods, modification of the genome using most reprogramming protocols, and the lack of failsafe differentiation protocols for generation of functional differentiated phenotypes. Various non-integrating and thus safer methods of reprogramming have been achieved but these too come with their own limitations. In addition, the majority of these technologies require the exposure of cell nuclei to reprogramming large molecules via transfection, transduction, cell fusion or nuclear transfer that raises several technical, safety and ethical issues.
Chemical genetics is an alternative approach for cell reprogramming that uses small, cell membrane penetrable substances to regulate multiple cellular processes including cell plasticity. Several recent groundbreaking studies showed that this approach can been used to produce desired cell types via direct induction of somatic stem cells into other cell fates, termed lineage-specific reprogramming or via generation of pluripotent stem cells termed chemically induced pluripotent stem cells.
Recently, using a chemical genetics approach (the combination of small molecules that are involved in the regulation chromatin structure and function and specific cell signalling pathways, we have been able to generate neural-like cells (Fig.1.b,c) from human mesenchymal stem cells (hMSCs) (Fig.1.a). Neurally induced hMSCs (NI-hMSCs) express several progenitor and mature neuronal markers (Fig.1.b, Fig.2.a-t), exhibite electrophysiological properties of maturing neurons (Fig.3), and formed synapses with differentiated human neurons in co-cultures (Fig.1.c).

Fig.1. Turning human bone marrow derived MSCs (a) into neuronal cells (b,c). GFP positive Ni-hMSCs form synapses with adult human neurons in co-culture (c).
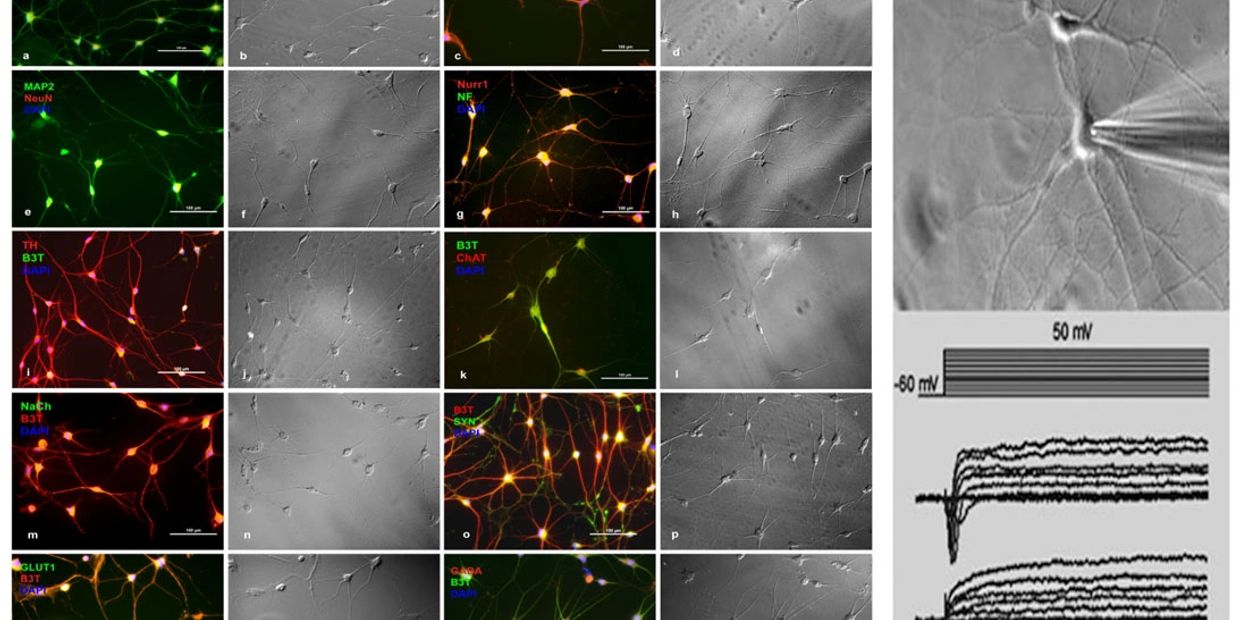
Fig.2 Morphological and immunocytochemical characterization of NI-hMSCs. Neurally reprogrammed cells expressed several neuronal progenitor and mature neuronal markers. For each fluorescence image, a phase contrast image was also captured. NI-hMSCs exhibited Na+ current (middle) and K+ currents (bottom) in response to depolarizing steps (Top, holding potential, -60 mV, 10 mV per step).
Recently using similar approach we have been able to generate neuronal subtypes from human adult fat cells
The main objectives of the Company are: 1) optimization of these new technologies to generate relatively pure population of neuronal subtypes and to produce clinical grade cell-based products; 2) to elucidate the therapeutic effects of these specialized neuronal cells in several nourological disorders such as Parkinson's, Alzheimer's, Huntington's, ALS, Stroke, and CNS injuries; 3) to use these neuronal cells for the development of cell-based high throughput assays for screening of neuroprotective and neurotoxic agents.
Commercial and clinically compatible products emerging from thesestudies include different neuronal differentiation kits, technologies for large-scale clinical grade production of these cells and high throughput neurotoxic / neuroprotective screening systems, provided as a service by Cell Reprogramming & Therapeutics LLC or customized for end-user internalization.
Copyright © 2024 Cell Reprogramming & Therapeutics LLC - All Rights Reserved.